


CENOS is our innovative platform technology which comprises of our portable instrument and Extraction Free (XF) Reagent System.
CENOS has the unique ability to carry out high sensitivity RT-qPCR diagnostic testing direct from almost any crude sample medium (including whole unprocessed blood) anywhere in the world without the need for expert personnel, lab facilities or equipment whilst the patient (human or animal) waits .....and in under 30 minutes. CENOS embodies and supports our strategy of At Patient Testing (APT).

Key Benefits
- At Patient Testing (APT) in under 30 minutes from test to result whilst the patient waits (both human and animal) - APT represents true point of care testing without frontiers or limitation
- Portable platform weighing under 2kg means it can deployed into almost any environment including in-field use in decentralised settings
- Simple to use with no requirement for specialist equipment, expert users or complex cartridge based consumables
- Low cost per test makes CENOS accessible to all users and applications including veterinary, Viral Haemorrhagic Fevers (VHF’s) and testing in resource poor environments often representative of Low to Middle Income Countries (LMIC’s)
- Sensitive gold standard RT-qPCR that meets LLOD reference tests enabling the early detection of subclinical, asymptomatic and non-symptomatic cases
- Extraction Free (XF) Reagent System enables detection direct from crude unprocessed samples (including whole blood) without the need for complex and time consuming nucleic extraction processes
- Cold-chain free means there is no requirement for specialist storage conditions and supports testing in-field in decentralised settings
- Multiplex detection supports differential diagnostics (up to 6 targets for blood borne and respiratory panels)
- 1-8 channels can be configured using the modular CENOS systems and THEIA software
- Intelligent automated results calling using a simplified traffic light system provides accurate and clear results which can easily be interpreted by any user
- Durable and robust design means CENOS can be safely transported and used in-field in unforgiving environments
- Internal positive controls based on Armoured RNA provides complete confidence in results
- Flexible open platform where new tests can be quickly developed, optimised, validated (in accordance with our certified ISO 13485 quality management system) and deployed at scale (250,000 tests per week) in approximately 6 weeks in response to an emerging outbreak
In-Field Sampling in Under 30 Minutes
The CENOS platform consists of a field portable instrument for the automated analysis of the molecular test and proprietary lyophilised reagents (cold-chain free) capable of performing RT-qPCR based pathogen detection. CENOS is truly unique in that non-expert users can perform gold standard RT-qPCR direct from crude samples (including unprocessed blood) in-field whilst the patient waits in under 30 minutes from test to result representing true At Patient Testing (APT).
Rapid Deployment
CENOS can be rapidly deployed anywhere in the world and effectively used in decentralized settings with minimal infrastructure (off-grid powered) and resources for large scale in-field testing. The modular, generic and simplistic nature of the system offers a high degree of flexibility and adaptability, which serves as an open platform. These unique capabilities are paramount when responding to emerging outbreaks to identify pre-symptomatic and asymptomatic individuals at the earliest stage of and source of infection. This will improve patient outcomes, triaging and containment supporting the breaking of transmission chains helping to prevent future pandemics.

The CENOS system is designed to be used as the first line of defence in conjunction with RT-qPCR lab based testing (for high throughput and sensitivity screening focused on population health), Lateral Flow Testing (for mass population screening) and vaccination programmes as part of a holistic and multi-faceted strategy to effectively combat High Consequence Infectious Diseases (HCID).
Cold Chain Free
Our proprietary molecular diagnostic reagents are cold-chain free and supplied ready to use in our dedicated consumable. Users simply add the provided resuspension buffer and their chosen sample medium to perform the closed tube reaction.
Being cold-chain free there is no requirement for specialist storage facilities or transport conditions. This means our tests can be deployed and used anywhere in the world. Lyophilised reagents and hot-start aptamer technology allow reactions to be set-up at up to 40C with no adverse effect on performance or risk of denaturation. This makes the system perfectly suited for in-field testing in almost any environment from Europe to warmer climates such as Africa, South America, Southern Asia and inevitably other regions of the world subject to the impact of climate change.
Extraction Free
Molecular tests currently available typically rely on nucleic acid extraction, performed by an expert user. This process restricts testing to laboratory based facilities and field hospitals. Samples are often transported to these facilities and batch tested through high throughput automation which means long turnaround times and an increased risk of transmission whilst the patients wait.
Our novel reagents are capable of detection from a wide range of crude samples without the need for nucleic acid extraction and purification. This includes whole blood (plasma/serum), saliva, nasal/oral/ocular swab, viral transport medium, cerebrospinal fluid and urine. Non-invasive sampling methods can also be used such as finger-prick testing in place of venous draw methods. As a result our reagent system can be deployed in response to a diverse range of viral pathogens due to its universal adaptability.
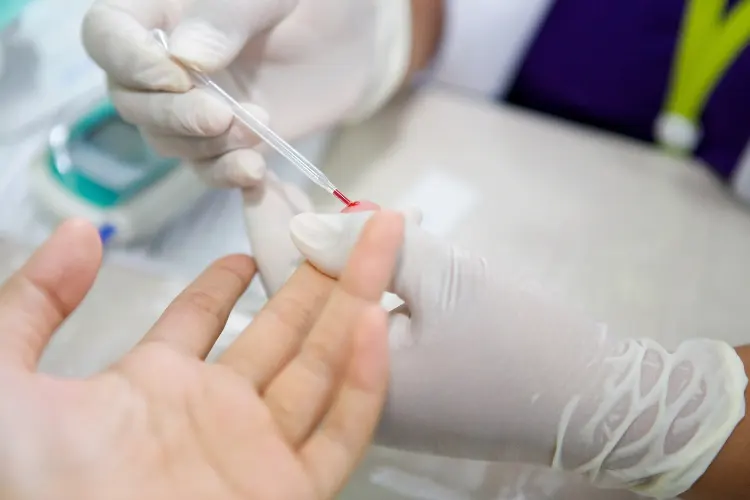
Removing the requirement for nucleic acid extraction negates the need for complex and expensive plastic cartridges, specialist laboratory facilities, equipment and personnel. Being a 1-step closed-tube reaction it reduces the risk of user error and infection when handling samples which negates the need for a protective containment hood. This makes CENOS perfectly suited to in-field testing with zero reliance on specialist facilities, equipment and personnel.
Our extraction free process has been made possible through the development of a single thermostable specialised enzyme and bespoke optical system that can tolerate typically inhibitory sample types such as whole blood to perform gold standard RT-qPCR. This represents a truly unique and powerful application of molecular diagnostics.
Rapid Multiplex Differential Diagnostics
CENOS is capable of performing multiplex RT-qPCR which includes integrated controls for verification of results. This enables low cost and rapid differential diagnostics for pathogens displaying similar symptoms.
The system uses sensitive real-time optics based on spectrophotometry to optically interrogate the sample to detect and differentiate between fluorescent dyes, each representing a chosen target pathogen.
CENOS is an open access platform where new assays can be quickly developed, optimised and run. We provide a rapid assay development and in-house validation pipeline approach that enables new in-field assays to be deployed at scale in response to High Consequence Infectious Diseases (HCID) and future outbreaks. Our assays can be configured and optimised for specific sample mediums and multi-plex panels (respiratory and blood borne) in accordance with user requirements.
Simple and Cost Effective Testing
CENOS has been developed with the goal of improving global access to gold standard RT-qPCR diagnostics in decentralised settings and to ultimately save lives. To make this possible the platform has been designed so it can be both simplistic and cost effective. This makes CENOS accessible to all users and applications including veterinary, Viral Haemorrhagic Fevers (VHFs) and testing in resource poor environments often representative of Low to Middle Income Countries (LMICs).
Currently there are only “near patient” tests available which use complex cartridge based systems to automate the nucleic acid extraction process. This approach results in a high cost per test, plastic wastage, not cold-chain free and requires an expert user to operate making them unsuited to in-field testing.
CENOS does not require nucleic acid extraction so there are no complex cartridges, moving parts, magnetics or costly microfluidics required. It is simply comprised of our dedicated consumable and lyophilised reagent set.
The simplicity of CENOS means the system is inexpensive and the cost per test is low in comparison to our competitors – none of which are capable of performing true At Patient Testing (APT).
CENOS uses intuitive automated results calling algorithms which correlate Ct values to clinical metrics. The result output is displayed as a simple traffic light system that interprets results as either ‘Positive’, ‘Negative’ or ‘Repeat’ ensuring complete unambiguity and confidence regardless of the users expertise.
Find Out More
To request a demonstration of CENOS or to find out more, please get in touch.




